
Introduction
Polystyrene is a hard, brittle plastic. It’s used for many things like plastic containers and utensils. Polystyrene will be made using free-radical polymerization of the monomer, styrene. Benzoyl peroxide will be the radical initiator and the solvent is toluene.
Purpose
Free-radical polymerization of Styrene to yield Polystyrene.
Data:
Toluene is a good solvent because it’s soluble and it will initiate addition of the polymerization chain. Methanol is a good solvent because it’s able to lower its solubility to form the precipitate.
| Toluene (Solvent) | Benzoyl Peroxide (Radical initiator) | Methanol | Styrene | Polystyrene | |
| Chemical Formula | C6H5CH31 | C14H10O42 | CH3OH3 | C6H5CHCH24 | (C8H8)n |
| Molecular Weight | 92.141 g/mol1 | 242.23 g/mol2 | 32.042 g/mol3 | 104.152 g/mol4 | |
| Density | 0.9 g/mL | ||||
| Amount Used | 6mL | 0.12g | 150mL | 4mL | |
| Mass Obtained (g) | 3.6 g | 2.2982 g |
| IR Value | Bond and Intensity |
| 3371 cm-1 | O-H weak, broad |
| 3026 cm-1 | Sp2 C-H stretch weak |
| 2919.8 cm-1 | Sp3 C-H stretch weak |
| 1603.3 cm-1 | Aromatic C=C stretch weak |
| 1497.1 cm-1 | Aromatic C=C stretch medium |
| 1452 cm-1 | Aromatic C=C stretch medium |
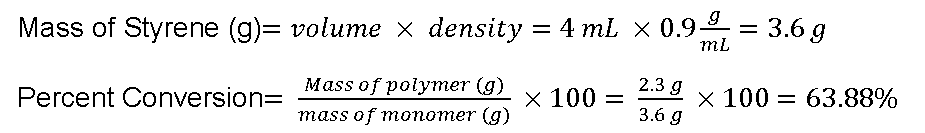
Procedure
Place 6 mL of Toluene and 4 mL of Styrene in a 50 mL round bottom flask. Then add 0.12 g of Benzoyl Peroxide and swirl. Set up for reflux for 30 minutes then cool. Add the mix slowly with constant stirring to a beaker containing 150 mL of Methanol. Collect precipitate by vacuum filtration. Transfer to weigh boat. Collect weight and IR.
Discussion
Polymerizing the monomer styrene by using free-radical polymerization will yield polystyrene. By adding 4 mL of styrene to a mix of 0.12g of benzoyl peroxide, the radical initiator, in 6mL of the solvent toluene yields 2.3 g of polystyrene. To calculate how much of the monomer is incorporated into the polymer percent conversion is calculated. By dividing the mass of the polystyrene and the mass of styrene results in a 63.88% conversion. This indicated that 63.88% of the styrene was used to make the polystyrene which is relatively low compared to how much styrene we used.
The results from the product IR are similar to styrene’s. Both have small peaks after 3000cm-1 indicating Sp2 carbons in the aromatic. The signals at 1452, 1497.1, and 1603.3 are also indicators of aromatic C=C stretches. One major difference is that in the polymer a Sp3 carbon is formed, indicated by the medium peak at 2919.8cm-1, because there is no longer an alkene. Another major difference is the elimination of the alkene C=C stretch at 1630.06cm-1 on styrene’s IR spectrum from the polystyrene IR. The presence of a broad signal at 3371cm-1 indicates an alcohol. This could be leftover methanol from our experiment.
The precipitate was white and solid.
References
1Toluene https://pubchem.ncbi.nlm.nih.gov/compound/toluene#section=Top (9/12/2018)
2https://pubchem.ncbi.nlm.nih.gov/compound/benzoyl_peroxide#section=Top (9/12/2018)
3https://pubchem.ncbi.nlm.nih.gov/compound/887(9/12/2018)
4https://pubchem.ncbi.nlm.nih.gov/compound/7501#section=Top(9/12/2018)



